Strong Alkali Examples
I Reaction of Base with Metals. Super Base Deprotonation is more efficient when using these bases instead of a strong one.

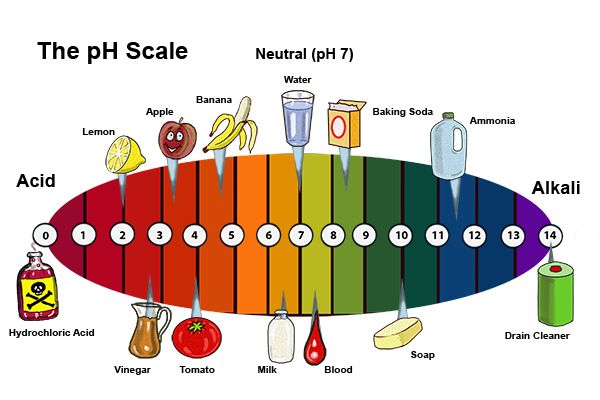
List Of Strong Bases 7 Examples Chemistry Teachoo
While most metals are hard lead and gallium are examples of elements that.

. The hydroxide of alkali and alkaline earth metals are soluble in water. What is meant. One allotrope of tin behaves more as a nonmetal.
K w H OH. For example Sodium lauryl sulfate Sodium dodecylbenzene sulphonate and so forth. Larger atomic size of alkali metals indicates that there is a less attractive force between the nucleus and outermost electron.
Ammonia NH 3 water H 2 O pyridine C 5 H 5 N are examples. Most of the nonpoint sources have been initially recognized as such by groundwater experts Foster et al 2003 who realized that soil urban or rural was an important means of transporting pollution to ground. Chemistry the science that deals with the properties composition and structure of substances defined as elements and compounds the transformations they undergo and the energy that is released or absorbed during these processes.
The Leonhardskirche also a Gothic building of the 15th century. Every substance whether naturally occurring or artificially produced consists of one or more of the hundred-odd species of atoms. As well as the weak base the aqueous solution contains its conjugate acid.
They are bitter to taste if placed in alkali solutions. Long-chain alcohols are treated with strong sulphuric acid and then neutralized sulfate with alkali to make synthetic detergents. Alkaline earths are reactive but less so than alkali metals.
The equilibrium constant for this reaction defined as. Alkali metals and conjugate acids can be mixed to produce these compounds. Of the numerous churches in the city the most interesting are the Stiftskirche with two towers a fine specimen of 15th-century Gothic.
Sodium hydroxide magnesium hydroxide calcium hydroxide etc. A chemical element that is a common greyish-coloured metal. Due to less attraction force the alkali metals show a tendency to lose electrons easily.
Has a value close to 10 14 at 25 C so the concentration of hydroxide ions in pure water is close to 10 7 moldm 3 in. Alkali is considered a strong base. Chemical properties of bases.
These are also known as alkali. They form hydroxide ions and increase the concentration of hydroxide in the water. The hydroxide ion is a natural part of water because of the self-ionization reaction in which its complement hydronium is passed hydrogen.
A base is a substance that can neutralize the acid by reacting with hydrogen ions. There are many reasons behind the reactivity of alkali metals. Like the alkali metals these elements are found in compounds rather than pure form.
H 3 O OH 2H 2 O. Examples of bases are sodium hydroxide calcium carbonate and potassium oxide. The Hospitalkirche restored in 1841 the cloisters of which contain the tomb of Johann Reuchlin.
Soaps are widely used in bathing cleaning washing and in other household chores. Let us discuss them one by one. The adjective alkaline and less often alkalescent is commonly used in English.
In chemistry an alkali ˈ æ l k ə l aɪ. Saponification - Saponification is the hydrolysis of an ester with NaOH or KOH to give alcohol and sodium or potassium salt of the acid. The fine modern Gothic church of St John.
Some examples of common products that contain Arrhenius bases include. It is strong used in making steel. Their conjugate acids are very weak.
Most bases are minerals that react with acids to form water and salts. The new Roman Catholic church of St. Ashes of the saltwort is a basic ionic salt of an alkali metal or an alkaline earth metalAn alkali can also be defined as a base that dissolves in waterA solution of a soluble base has a pH greater than 70.
When alkali base reacts with metal it produces salt and. Visit BYJUS to know the saponification process saponification reactions saponification value with Videos and FAQS in detail. Group IIA metals are hard and shiny and usually malleable and ductile.
1 Large atomic size. Water pollutants come not only from urban and municipal wastewater discharges but also from nonpoint sources some of which are not perceived as such. Strong or concentrated bases are caustic.
Named for Swedish scientist Svante Arrhenius to describe base behavior in water these bases dissociate when added to an aqueous solution making them strong bases. And combinations for soakingpre-washing rinsing or bleaching are examples of common detergent products.
No comments for "Strong Alkali Examples"
Post a Comment